
from the LIME team and on the following topic:
"Speciation of Molybdenum in Uranium Dioxide: Impact on Dissolution Kinetics under Reprocessing Conditions".
Defense scheduled for Friday, November 8, 2024 at 2:00 PM (ICSM Auditorium).
The UOx spent nuclear fuel (SNF) is a complex heterogeneous system in terms of microstructure, chemical composition, and elemental distribution. SNF dissolution in hot concentrated nitric acid constitutes the head-end operation of reprocessing by the PUREX process. The fission products (FPs) that occur in many different phases depending on irradiation conditions can influence the dissolution kinetics of the SNF. Understanding the impact of the main FPs and actinides on SNF dissolution kinetics is important in order to improve our ability to anticipate the behavior of the SNF during reprocessing. The aim of this research work is to investigate the impact of Mo, one of the most abundant FPs, on the dissolution kinetics of UO2 in nitric acid under representative PUREX process conditions. In the SNF, Mo has a complex speciation linked to its interaction with the fuel and other FPs. It can be found in different oxidation states (0, +4, and +6) in metal inclusions or oxides under specific irradiation conditions. Even after SNF dissolution in nitric acid, Mo is a significant component of various undissolved residues that complicate the recycling processes.
In this context, simplified UO2-based model compounds incorporating different contents of Mo in the metallic state (prevailing state in SNF) were first prepared by hydroxide precipitation followed by shaping and then sintering at a high temperature under a reducing atmosphere. Dense pellets with controlled physico-chemical properties and a representative microstructure of SNF were produced. Another set of UO2-model compounds incorporating mixed Mo phases, Mo0 and BaMoIVO3 (analog of the gray perovskite phase), were also prepared by successive hydroxide precipitation steps followed by conversion at a high temperature under a reducing atmosphere. The speciation, morphology, and spatial distribution of the elements in the converted samples were determined. Dissolution experiments were carried out under representative SNF reprocessing conditions (4 mol L-1 HNO3 at 80°C) following a dynamic protocol. The dissolution of pure Mo0, MoIVO2, BaMoIVO3, and BaMoVIO4 reference samples was also carried out under the same conditions to understand separately their dissolution mechanisms in nitric acid and evaluate their influence on UO2 dissolution kinetics. The obtained results provide essential insights into the dissolution mechanisms of the different Mo phases and the specific role of the Mo oxidation state in UO2 dissolution in nitric acid, offering potential pathways for optimizing the recycling process.
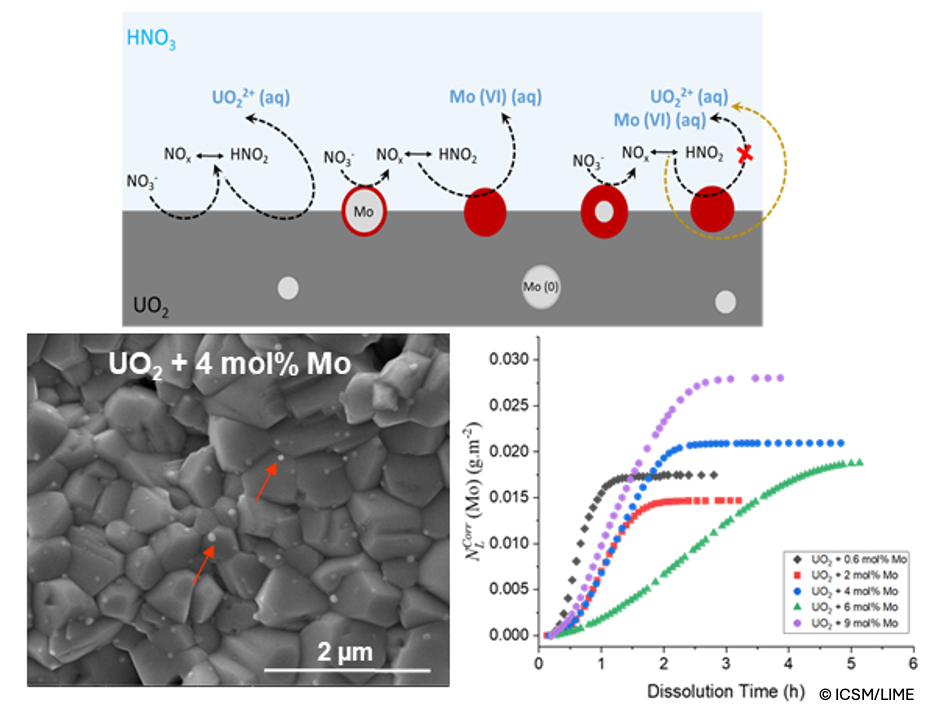
Keywords: Molybdenum; UO2-based model compounds; Dissolution mechanisms; Solid-liquid interface; Nuclear fuel recycling