
from the LMCT team and on the following topic:
"Molecular prediction of Gibbs energies of ion transfer for liquid-liquid extraction "
Defense scheduled for Tuesday, December 2, 2025 at 2:00 PM (ICSM Auditorium).
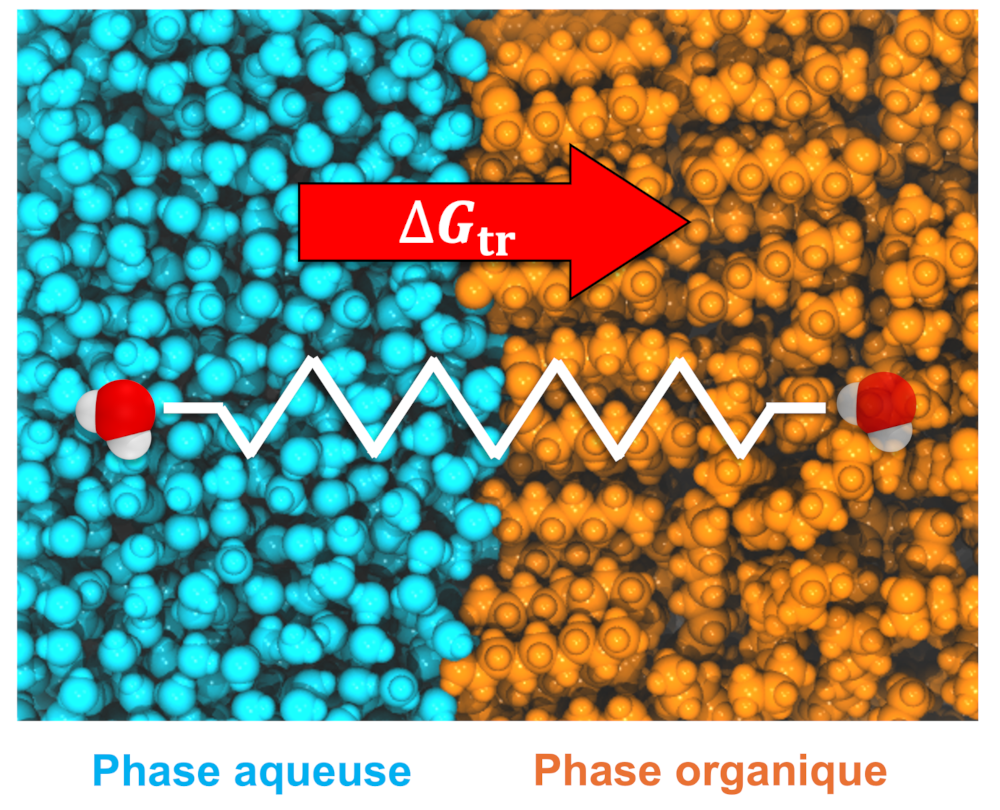
This thesis provides a method for estimating the Gibbs energy of transfer based on Steered Molecular Dynamics (SMD) biased simulations. This method was developed and applied to liquid-liquid extraction systems, from organic-phase simulants to aqueous/organic phase interfaces. It is supplemented by an in-depth molecular dynamics analysis of the structural properties of these multiphasic systems. The demixing of binary and ternary water/alcohol mixtures was first investigated, with phase identification performed using graph theory and theoretical reconstruction of X-ray scattering spectra. Subsequently, the speciation of lanthanum nitrate salts (La(NO3)3) in methanol was studied. Simulations revealed the formation of polynuclear complexes, whose structure and aggregation thermodynamics were characterised. Then, the study of water/octanol systems investigated the presence of octanol bilayers at the interfaces. The SMD methodology was developed in these systems, accurately predicting the solubility of water in octanol in agreement with experimental data. Finally, the energetic and structural analysis of aqueous/DMDOHEMA/dodecane systems demonstrated the influence of extractant and La3+ concentrations on interfacial structure and on the transfer free energies of water and La3+ cations. The findings of this study present new insights for the modeling of liquid-liquid extraction systems by reconciling structural property analysis with thermodynamic prediction by means of SMD simulations.
Credit: ICSM/LMCT
Keywords: Molecular Dynamics; Steered Molecular Dynamics; Liquid-liquid extraction; Interfaces; Structural properties; Gibbs energies of transfer