
from the LMCT team and on the following topic:
"Simulation of the equilibrium and the transport of ions at the liquid-liquid interfaces"
Defense scheduled for Thursday, November 20, 2025 at 2:00 PM (ICSM Auditorium).
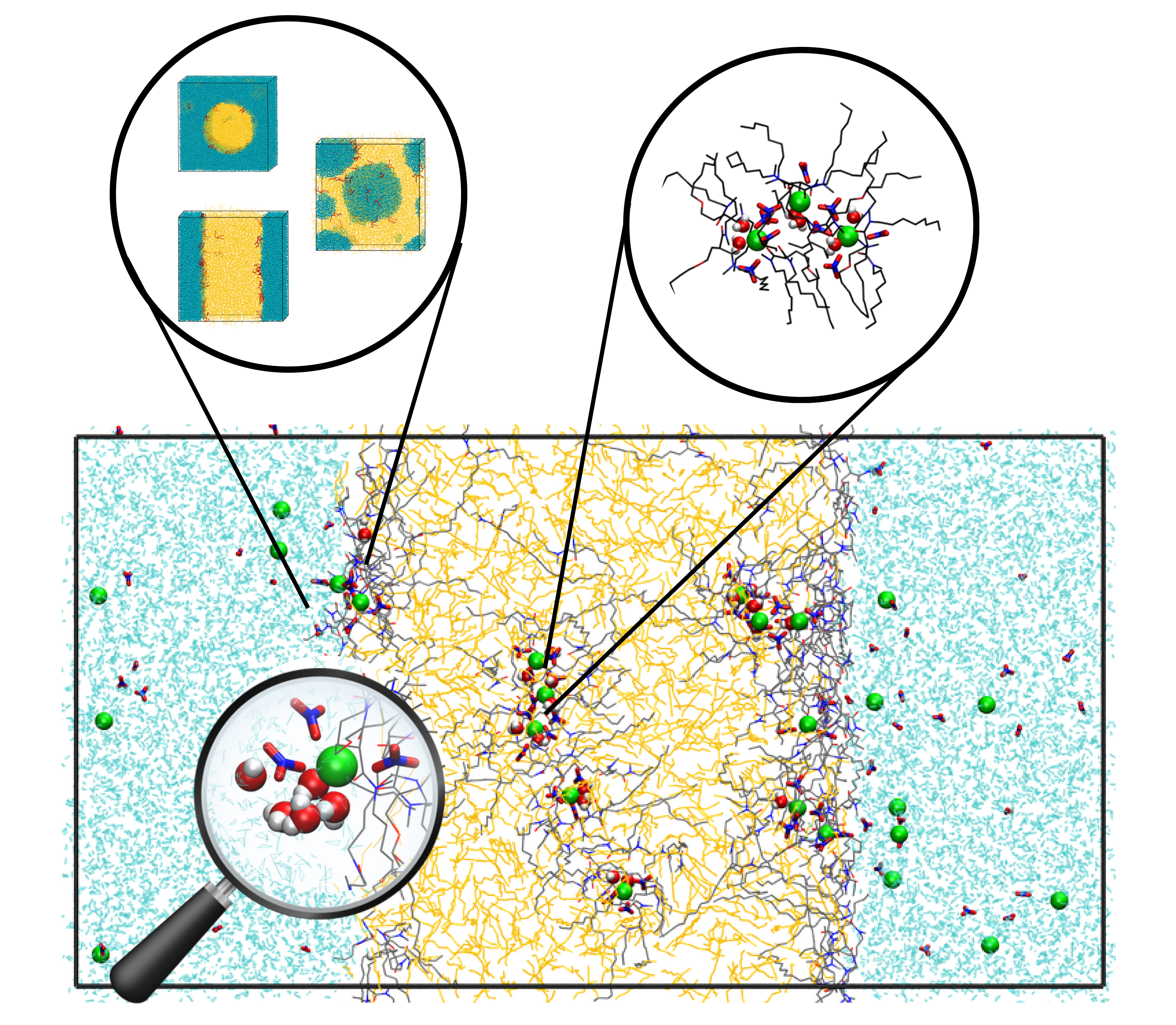
In this thesis, the structural organization in the bulk organic phase, at the interface, and the transport of ions in systems with the extractants DMDOHEMA, DMDBTDMA, and TODGA were investigated using classical molecular dynamics. First, we demonstrated that using a demixing approach, i.e., starting from randomly distributed molecules, was the optimal approach to create interfaces. The analysis revealed different interfacial shapes depending on the ratio of the two immiscible liquids, with the organization of extractants directly influencing interfacial thickness. We then focused on bulk organic phases containing Eu(NO3)3 salt in the presence of the three extractants. To characterize the aggregates formed in the organic phase, the code CoordDynA_MD was developed. All extractants form reverse micelle-type aggregates, with compositions depending on the extractant used. We demonstrated that increasing the Eu(NO3)3 concentration impacts the number of aggregates comprising more than one cation but not the average oligomer stoichiometries. These oligomers arose from monomeric aggregates connected through hydrogen bonds involving water molecules, nitrate anions, and extractant molecules, which enabled the formation of bridges. Finally, the transfer of Eu(NO3)3 from the organic phase to the aqueous phase was studied. The mechanism, driven by nitrate-water H-bonding in the aqueous phase and the cation hydration, was independent of the extractant. However, distribution ratios and retention times varied, with shorter retention times for extractants that were able to form larger aggregates.
Credit: ICSM/LCMT
Keywords: Liquid-liquid extraction; Interfaces; Molecular Dynamics; Lanthanides