
from the LTSM team and on the following topic:
"N,P ligands for actinide extraction"
Defense scheduled for Thursday, October 30, 2024 at 9:00 AM (ICSM Auditorium).
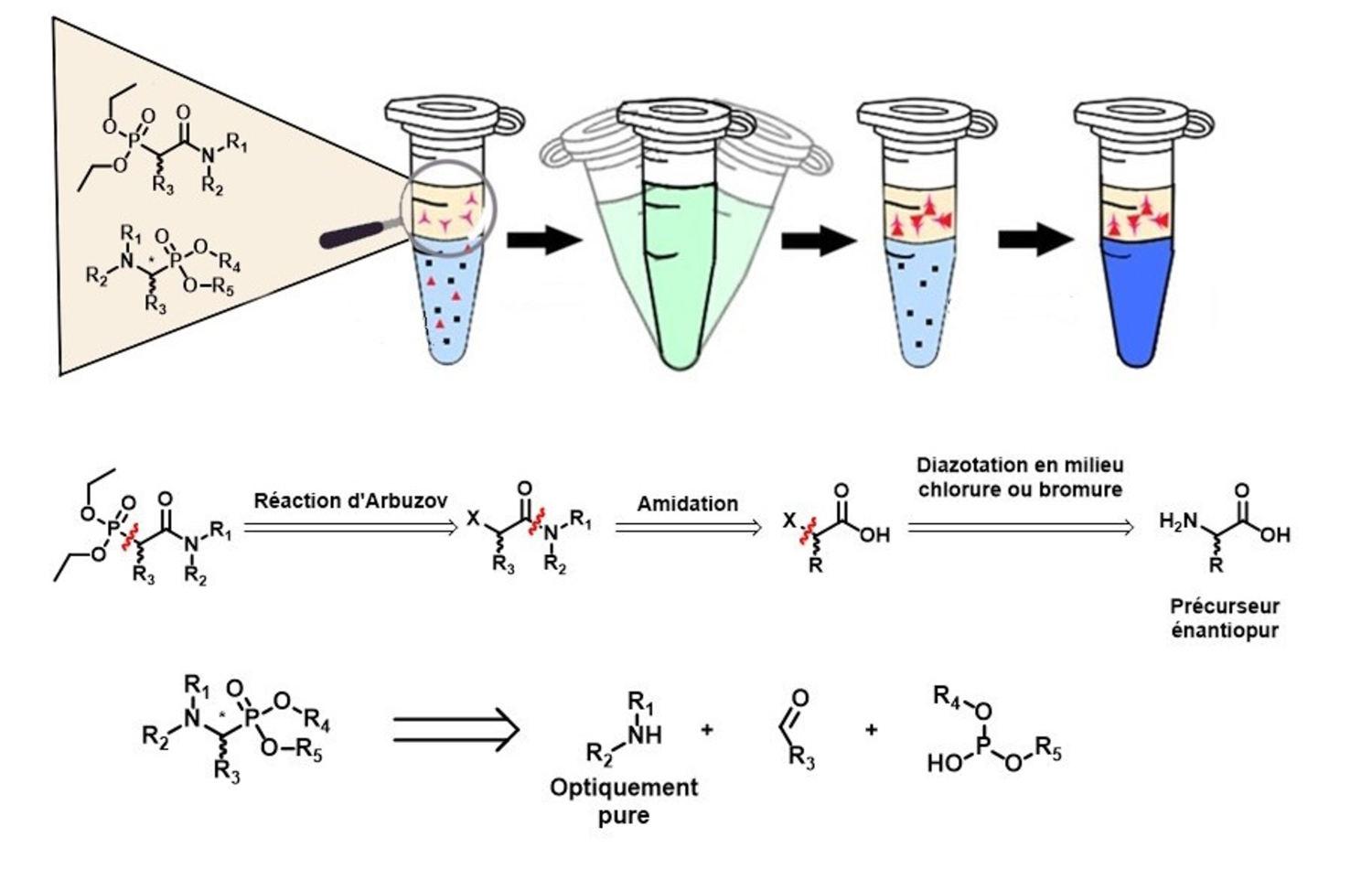
The nuclear fuel cycle involves considerable challenges for the future of nuclear power. Among the hydrometallurigical processes used for metal extraction and recycling, such as for uranium, liquid-liquid extraction is one of the most commonly used techniques. Bi-functionnal ligands such as amido- and aminophosphonates are recognised as effective specific uranium extractants. Several studies have demonstrated that the stereochemistry of an extractant influences metal extraction. Using one diastereoisomer rather than another, or a mixture of isomers, can significantly alter extraction efficiency. In this context, the aim of this thesis is to develop methodologies for the asymmetric synthesis of chiral amido- and aminophosphonates. Three pathways were studied for amidophosphonates, By the end of this study, it had been demonstrated that the total synthesis from an amino acid precursor was the only approach for obtaining stereo-enriched amidophosphonates. Regarding aminophosphonates, the stereoselective Kabachnik-Fields reaction assisted by a microwave heating was studied. The influence of the regioselectivity of the chiral amine on the stereoselectivity of the reaction was also examined. These diastereoisomers were subsequently studied in liquid-liquid uranium extraction from nitric acid in order to investigate the effect of their stereochemistry on extraction efficiency. The results confirm that the stereochemistry of the ligands affects the extraction process. It appears that steric hindrance limits the formation and stability of the complexes.
Keywords: Asymmetric synthesis; ligand; chirality; hydrometallurgy; amidophosphonates; aminophosphonates; uranium
Credit: ICSM/LTSM
Keywords: Asymmetric synthesis; ligand; chirality; hydrometallurgy; amidophosphonates; aminophosphonates; uranium