
from the LNER team and on the following topic:
"Solid solution synthesis of actinide mixed oxides by Solution Combustion Synthesis".
Defense scheduled for Tuesday, December 19, 2023 at 9:00 AM (Amphithéâtre N°3, IUT Nîmes).
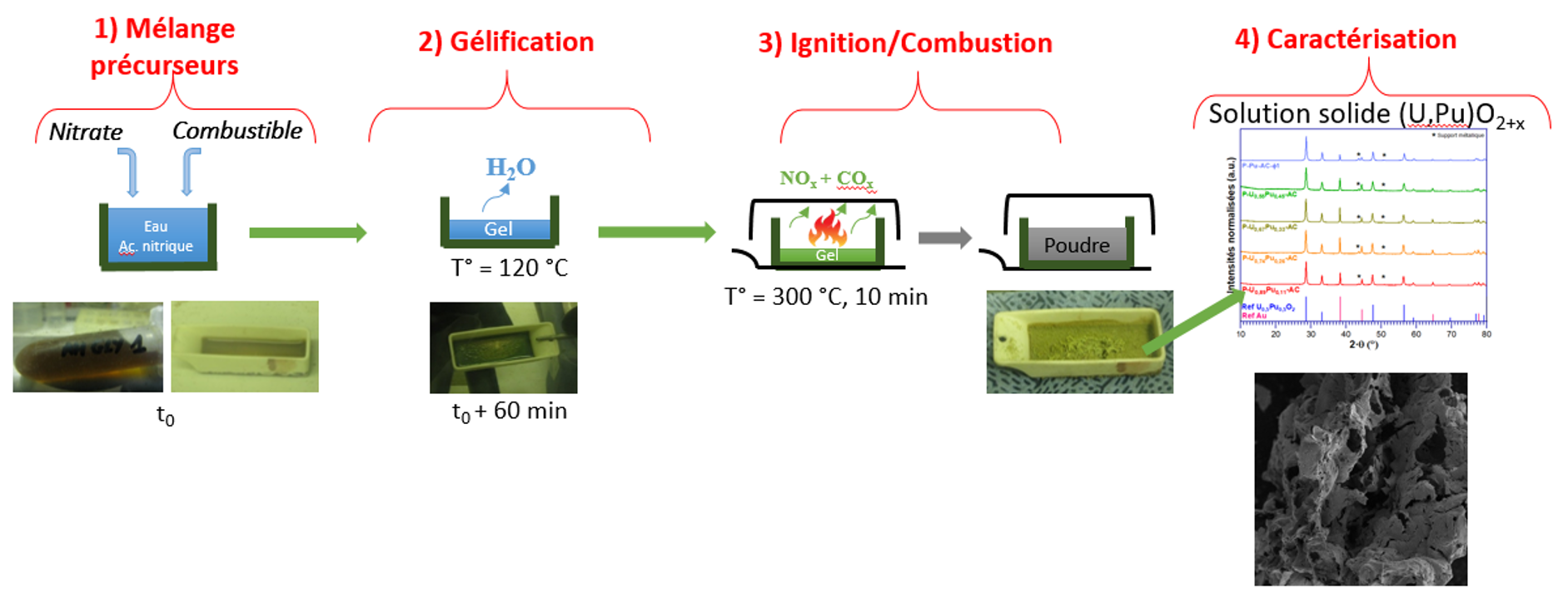
This work is part of research into the treatment and recycling of nuclear spent fuel. After dissolution, the conversion operation consists of manufacturing a new fuel from the nitric solutions obtained. Various conversion methods have been used to produce mixed actinide oxides: precipitation-calcination processes, impregnation processes, and, finally, thermal denitration processes using different heating methods. These methods produce powders whose characteristics can make it difficult to shape them into fuel pellets. Solution Combustion Synthesis (SCS), which consists of reacting a reducing organic compound called fuel with an oxidizing metal nitrate, leading to a highly exothermic reaction (ignition) at low temperature, typically 200-250°C, appears to be a promising process for obtaining a mixed oxide of uranium and plutonium with good chemical homogeneity and sinterability.
Credit: A. Hautecouverture / ICSM
In general, it has been shown that SCS leads to the formation of oxide particles with a size determined by the ignition and combustion process. Powder characteristics such as crystallinity, agglomeration, specific surface area, and crystallite size are highly dependent on the flame temperature reached during combustion. This temperature is governed by the nature of the reagents and the richness (oxidizer/reducer ratio) of the mixture. The aim of this thesis is twofold: to gain a better understanding of the reaction mechanisms at the origin of the SCS reaction, and to determine the optimum conditions for obtaining uranium, plutonium, and mixed (U,Pu)O2 oxides with the desired properties.
Firstly, a study was carried out on two actinide surrogate elements, gadolinium and cerium. The study of gadolinium nitrate with different fuels of varying carbon content and varying richness showed that even at optimum richness (stoichiometric conditions), the length of the carbon chain had a significant influence on the flame temperature and consequently on the characteristics of the oxide formed. The study on cerium nitrate demonstrated the feasibility of conversion to oxide over a wide range of richness, inducing differences in crystallinity. A study of the SCS of actinide oxides was carried out on uranium and then plutonium with citric acid and glycine, in order to identify the optimum conditions for conversion with these two fuels, identified in the study on simulants. Prior to this, spectroscopic studies of solutions and gels revealed differences in the speciation of these elements depending on the fuel used. The results obtained on the conversion of uranyl nitrate, cerium nitrate or plutonium nitrate served as the basis for a study on the synthesis of mixed oxides (U,Ce)O2 and then (U,Pu)O2. Citric acid was used to synthesize mixed oxides (U,Pu)O2+x, with different plutonium contents of industrial interest. The shaping and sintering steps in a reducing atmosphere were studied on UO2+x powders obtained by SCS. Several parameters were studied (fuel used for synthesis, post-calcination of the powder before shaping, pelletizing pressure) in order to optimize the process to produce a pellet of mixed oxide U0.89Pu0.11O2, reaching 88% of the theoretical density.
Keywords: Synthesis; combustion; actinides