
from the LIME team and on the following topic:
"Hydrothermal methods for the preparation and direct sintering of uranium-based mixed oxides".
Defense scheduled for Wednesday, December 13, 2023 at 1:30 PM (Visiatome Auditorium).
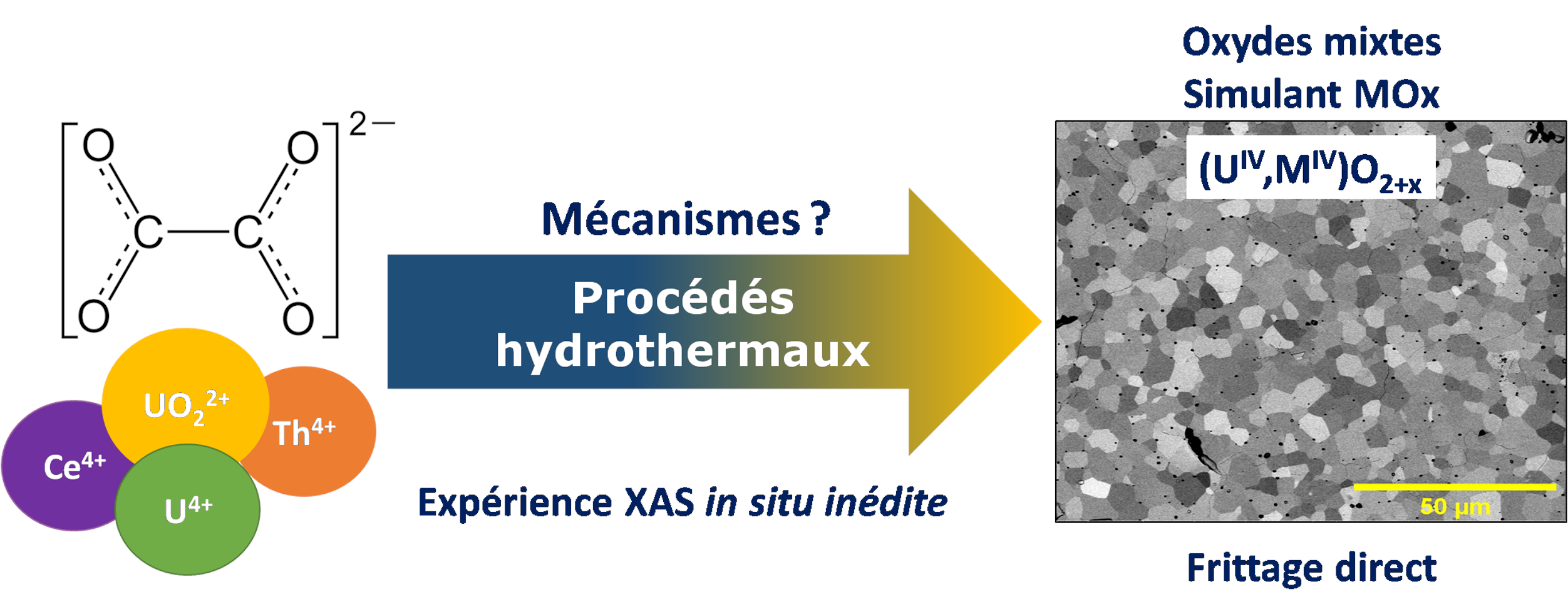
The development of new nuclear reactor technologies, known as generation III and IV, is leading us to consider innovative methods to manufacture MOx fuels (U,Pu)O2±δ. Wet processes are being examined in particular to improve the cationic homogeneity of powders and control their morphology. These methods are generally based on the precipitation of precursors at low temperature, followed by their conversion by heat treatment at high temperature. However, the resulting oxides may still contain traces of carbon, which is detrimental to ceramisation, and their morphology is often unsuitable for densification. This Ph.D. work investigates an alternative synthesis route for (U,Pu)O2 mixed oxides, by considering the hydrothermal conversion of model oxalate precursors based on U(IV)-Ce(III) and U(VI)-Th(IV) systems.
Credit: S. Benarib / ICSM
A multiparametric study of the hydrothermal conversion of mixed uranium(IV) and cerium(III) oxalates into mixed (U,Ce)O2+x oxides was first carried out at 250°C. The conversion was quantitative between pH = 2 and 10, with the acidity of the medium influencing the morphology of the oxides. The conversion was congruent and complete from 2h of treatment, with the cationic homogeneity of the oxides remaining unchanged afterwards. Finally, varying the cerium content altered the nature of the precursors and the time required to convert the oxalates. The optimum conditions for efficient conversion are therefore an initial pH = 8 and a duration of 24 hours. The hydrothermal conversion mechanism is also complex, involving several redox reactions and inducing a change in uranium valency within the oxides, corresponding to an oxidising dissolution followed by reductive precipitation. The sintering of mixed uranium and cerium oxide powders obtained by hydrothermal conversion was then studied in three stages. A dilatometric study highlighted the possibility of direct sintering, with densities reaching at least 92%TD. The oxides retain their structure and homogeneity throughout the temperature rise. However, de-densification phenomena were observed at 1700°C, probably due to the presence of residual carbon. Finally, sintering maps were constructed to predict the microstructure and density of the sintered oxides. Finally, the hydrothermal conversion of a solution containing uranium(VI) and oxalate ions was undertaken as a simplified model of the U(VI)-M(IV) system. The pH was identified as a key factor influencing the nature of the precipitated uranium phases. Optimum conditions for a complete conversion to UO2+δ were determined to a pH of 0.8 and a duration of 72h. Unprecedented in situ XANES analyses were carried out, allowing us to monitor uranium oxidation state within the hydrothermal reactor. The near-total reduction of uranium(VI) in solution was observed, prior to the precipitation of U(IV) in the form UO2+x·nH2O. Finally, the co-management of a two-phase reaction mixture consisting of uranyl ions in solution and thorium oxalate, in the presence of magnetic agitation within the reactor, enabled a solid solution of mixed U(IV)-Th(IV) oxide to be obtained. Hence, hydrothermal methods represent a reliable alternative for the synthesis of MOx fuels, with the possibility of co-managing actinides of different valences and chemico-physical forms.
Keywords: Hydrothermal methods; mixed oxides; uranium; sintering