
During the last JSUP 4 seminar (Ultrasound and Processes Days, Toulouse, 4-5 July 2017), the scientific council decided to ask the support of the CNRS in order to transform JSUP into a GDR ("Groupe de Recherche"). Sections 13 and 14 of INC and 10 of INSIS responded favorably and GDR CAVITATION was created on January 1, 2019. The GDR is headed by a director, Serguei Nikitenko (Research Director - CNRS, ICSM), assisted by an assistant director, Jean-Yves Hihn (Professor - University of Franche-Comté, UTINAM).
The main missions of GDR CAVITATION are to promote knowledge advances of the chemical and physicochemical reaction mechanisms under the effect of ultrasonic or hydrodynamic cavitation, to widen the potential applications of the sonochemistry, to reinforce the interaction between the GDR member-teams through collaborative projects, assisting in the organization of annual meetings and thematic schools, as well as providing support to young researchers mobility. The scientific perimeter of the GDR CAVITATION has been defined to respond more specifically to the following challenges: foster transdisciplinary exchanges; exchange practical solutions; compare viewpoints from applications as more basic work; strengthen links between academic and industrial partners; give visibility to the French sonochemical community; promote the emergence of research projects at the national and international levels.
More informations available on the GDR website: http://gdr-cavitation.cnrs.fr/

During cavitation bubble implosions, extreme temperatures (> 5000K) are reached in their heart. They are the source of a light emission called "sonoluminescence" (SL). The spectrum of SL generally consists of a continuum which extends from the UV to the near IR on which are superimposed emission lines of excited species (OH•, Ar*, excited ions, etc.). The measurement of the SL spectrum allows to probe the interior of the bubbles, obtain information on the temperatures reached and the presence of excited species. This study focuses a better understanding of the mechanisms of sonochemical reactions in order to optimize the operating conditions and maximize the production of radicals or excited species. A multifrequency (20 kHz - 1 MHz) SL reactor has been developed at ICSM for this study. SL spectra (230-900 nm) can be measured under the real conditions of sonochemistry (control of the temperature, nature of the dissolved gas, etc.) with a parallel and time-resolved analysis of the products.
The spectroscopic study of the SL spectra demonstrated the formation of non-equilibrium plasma in the heart of the cavitation bubbles during their implosions. Its characteristics can be studied via modeling, if necessary with spectroscopy software, of the emission spectrum of the observed excited species (OH, C2, Swan bands, NH, etc.). This approach allows estimating the vibrational and rotational temperatures of these excited species according to the experimental parameters (ultrasonic frequency, nature of the dissolved gas, concentration, etc.). For example, in the presence of 10-3 - 10-2 M t-butanol in water, under Ar at 362 kHz, the vibrational and rotational temperatures of C2 are ca. 8000 and 4000 K respectively.
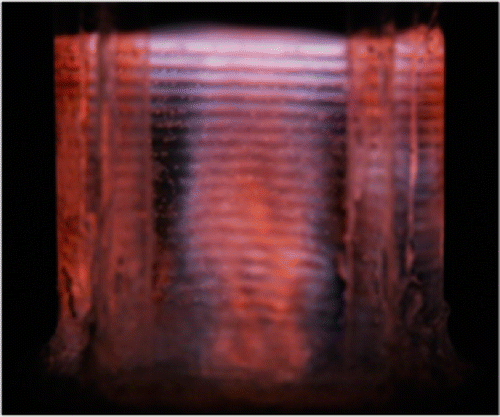
Fig. 1: SL of a NaCl solution.
In the nuclear industry, the decontamination of metal surfaces is of great interest for the dismantling of end-of-life facilities and equipments. The development of a set-up allowing the study of the behavior of extended surfaces under acoustic field allowed to demonstrate the possible decontamination of metallic surfaces artificially polluted by Ce, Sr, or U that can be envisaged in mild conditions (T, P ambiant) and with high decontamination factors. Sonochemical decontamination of Mg surfaces can thus be carried out by controlled depassivation and dissolution in order to eliminate only the desired thickness of metal (Fig. 2). The observations carried out by SEM demonstrate the structuring of the sample after treatment and the absence of residual contamination.
The contribution of ultrasound in decontamination has also been verified for dispersed solids in collaboration with CEA / DEN. Contaminated soil from Fukushima has been prepared as a surrogate within the form of vermiculite particles contaminated with cesium. In this type of material, Cs is trapped between the sheets of vermiculite, and decontamination generally requires ion exchanges (using high concentrations of Mg2+) and special conditions such as, for example, hydrothermal treatment. It has been shown that ultrasound allows gaining an order of magnitude on the desorption kinetics and increases the yields. It is planned to extend this technique to the remediation of soils contaminated with different metals.
The promising results observed in these domains can be envisaged on a larger scale where the technological possibilities linked to the use of power ultrasound in the industrial field are today varied and abundant.
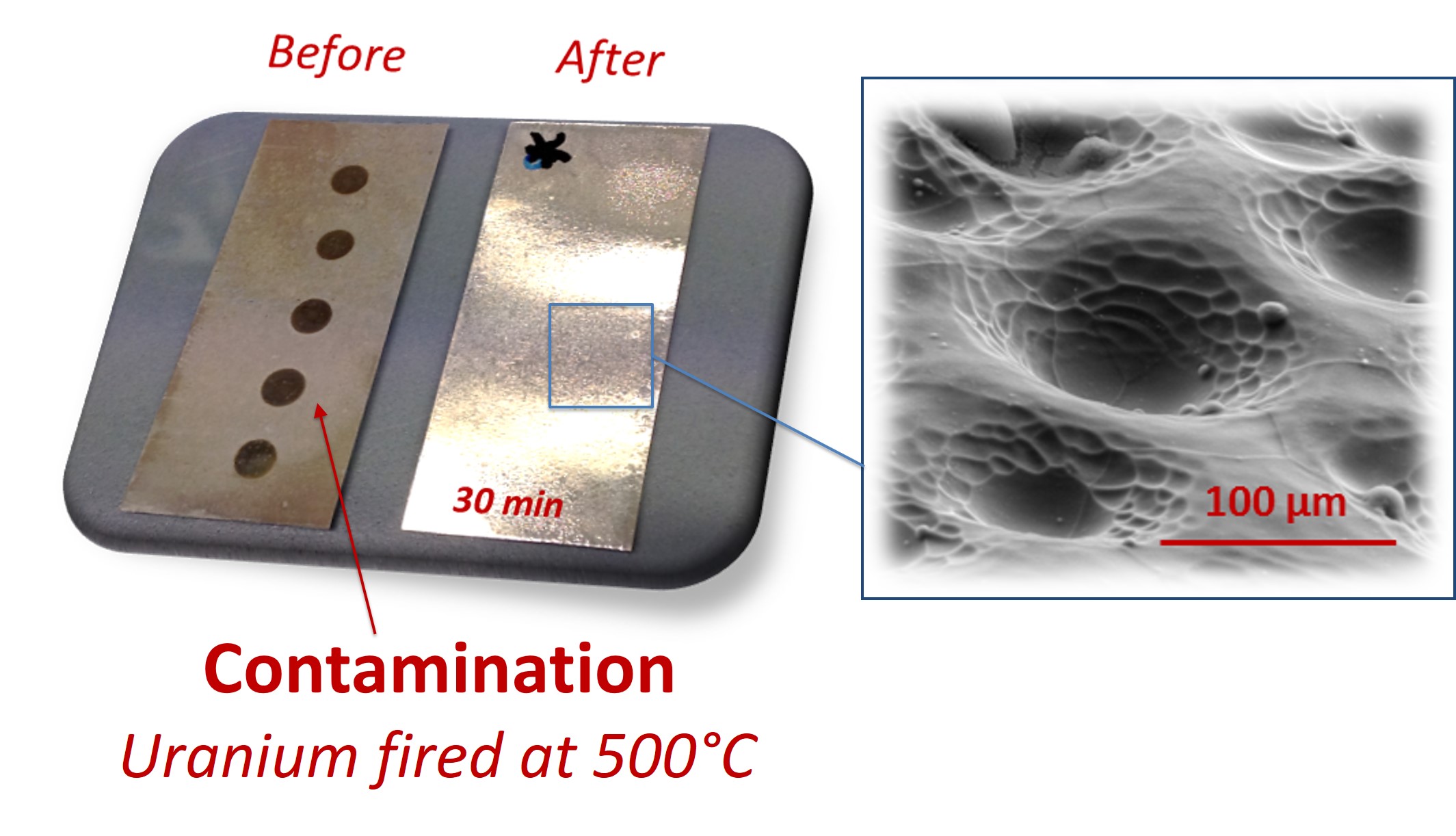
Fig. 2: Mg surface contaminated with U and treated during 30 min at 205 kHz in dilute aqueous solution (left). Local structuration of the surface observed with SEM (right).
Sonochemistry can be considered as a promising alternative for the reprocessing of current and future spent nuclear fuel due to the mild and efficient reaction conditions it carries. The associated acoustic cavitation phenomenon allows the in situ generation of redox species with controlled kinetics by working at ambient temperature and pressure, and without the addition of additional or concentrated reagents. The application of power ultrasound in actinide (An) chemistry where redox interactions are extremely frequent is therefore of great potential. The additional physical effects generated in heterogeneous solid-liquid systems lead to the observation of surface erosion and depassivation, grain fracturing, increased mass transfer and a decrease in diffusion layers. The sonochemical transformation of the reaction medium associated with the specific activation of surfaces can thus present many interests in the nuclear field. Within the framework of a solid collaboration with the DMRC department (Atalante installation, Marcoule, Fig. 3), several sonochemical set-up were nuclearised in a glove box in order to study the behavior of actinides under ultrasound irradiation. Three major research topics are investigated:
- Synthesis and redox control of actinides in aqueous solutions
- Synthesis, reactivity and characterization of actinide colloidal suspensions (Fig. 3)
- Reactivity and dissolution of actinide compounds (mainly oxides such as PuO2)
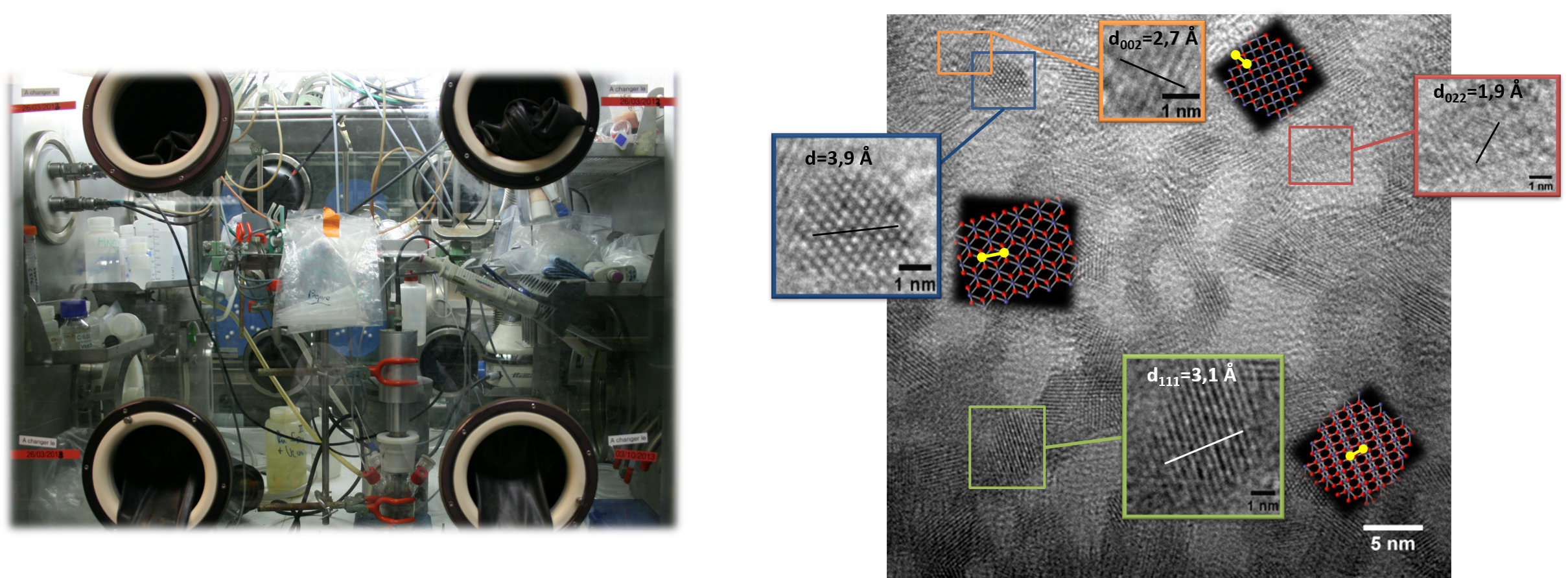
Fig. 3: Sonoreactor implanted in a glove box in Atalante facility (left) and HR-TEM image of Pu colloids (right).
Sonochemistry is a highly considered technique for the synthesis of various nanomaterials and catalysts. Carried out within aqueous media, sonolysis of water will lead to the formation of H atoms and OH radicals which can further recombine into hydrogen and hydrogen peroxide, respectively. In situ formed species can therefore be considered in order to initiate chemical reactions, especially the reduction of noble metals without addition of further reagents. High-frequency ultrasonic irradiation at room temperature can thus be considered for the preparation of catalysts through the sonochemical deposition of Pt, Pd or Pt-Pd nanoparticles on the surface of thermodegradable polystyrene beads and their transfer into a sol-gel derived porous silica matrix after the removal of the organic template (Fig. 4). The innovative method developed in our laboratory in collaboration with Montpellier, Delt and Messina Universities clearly demonstrates the ability to synthesize active and very selective catalysts of various compositions with tuned hierarchical porosity, high specific surface area and controlled active site localization. On the other hand, ultrasonic cavitation can be applied in a wide range of experimental conditions and even during hydrothermal treatment. The coupling of ultrasound with high temperature and autogenic pressure offers an opportunity to obtain new catalysts. For instance, a simple, easily scalable and environmentally friendly synthesis of stable Ti@TiO2 core-shell nanoparticles has been developed at 200°C under sonohydrothermal conditions.
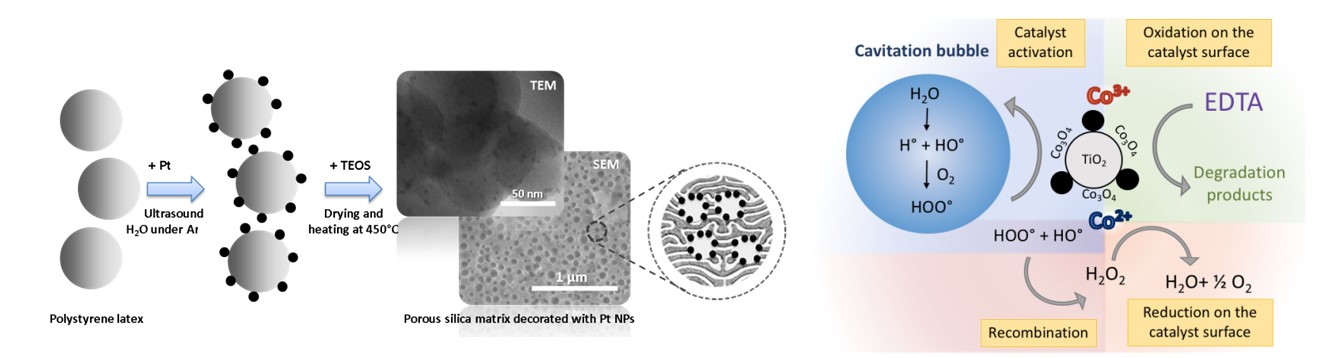
Fig. 4: Schematic representation of the Pt NPs deposition on the polystyrene beads and their transfer within a porous silica matrix (left). Mechanism of EDTA sonocatalytic degradation on Co3O4/TiO2 Catalyst (right).
The use of ultrasonic cavitation can also be a promising approach for sonocatalysis applications, namely the coupling of ultrasound and heterogeneous catalysis, especially from oxidative reactions and the degradation of organic pollutants. In fact, sonochemistry can be considered for wastewater treatment since OH radicals can be generated during water sonolysis. However, despite the extreme local conditions observed during acoustic cavitation phenomenon, and the generation of oxidizing and non-selective species, using ultrasonic irradiation alone is efficient only at low concentration in organic pollutants. Nevertheless, recent studies carried out in our laboratories pointed out that both the physical and chemical effects generated during the acoustic cavitation phenomenon can drastically enhance heterogeneous catalyst performances. Thus, in collaboration with IJLRA/ Sorbonne Université within the framework of the CADET project (ANDRA PIA), we demonstrated the complete defunctionalization and the partial mineralization of 5 mM EDTA aqueous solution within few hours under high ultrasonic frequency at 40°C in the presence of Co3O4/TiO2 catalyst and oxygen (mechanism in Fig. 4).