
Publication in Journal of Physical Chemistry Letters
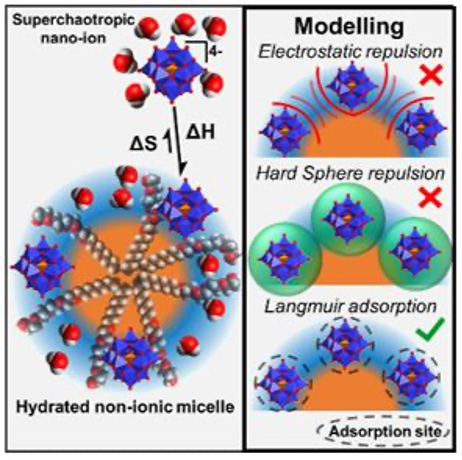
Team L2IAA salt (or its acid form) will be qualified as superchaotropic if its property of dissolution – the "salting-in" effect – or dispersion of a molecular or colloidal species is observable at concentrations of the order of a millimolar compared to a more conventional salt or chaotropic agent for which these salting-in effects are observable at much higher concentrations, of the order of a few tenths to a few molars. Among the chaotropic salts, we can mention, for example, lithium salts in bromide, perchlorate, thiocyanate form, and guanidine salts. Inorganic anions of nanometric size (or nano-ions) and low charge density, belonging to the family of polyoxometalates or boron ionic clusters have superchaotropic properties. Superchaotropy is linked to the weak hydration of nano-ions which gives them the ability to physically adsorb on molecules, surfaces, or non-ionic assemblies, thus strongly modifying their properties by stabilizing or destabilizing them depending on their formulation (concentration, salt, pH, presence of additives).
Recent works carried out at ICSM allow us to better understand this adsorption phenomenon by describing it with a thermodynamic theoretical model [1] (in collaboration with ICSM/LMCT). The control of molecular interactions by adding superchaotropic nano-ions offers practical opportunities that we are exploring to:
Credit: O. Diat / ICSM
For more information, read the articles:
[1] M. Hohenschutz, J.-F. Dufrêche, O. Diat and P. Bauduin. When Ions Defy Electrostatics: The Case of Superchaotropic Nano-ion Adsorption. Journal of Physical Chemistry Letters (2023), 14(15), 3602–3608. DOI: 10.1021/acs.jpclett.3c00095
[2] L. Braun, M. Hohenschutz, O. Diat, R. von Klitzing and P. Bauduin. Repulsive, but sticky – Insights into the non-ionic foam stabilization mechanism by superchaotropic nano-ions. Journal of Colloid and Interface Science (2023), 641, 437–448. DOI: 10.1016/j.jcis.2023.03.030
[3] I. Chazapi, O. Diat and P. Bauduin. Aqueous solubilization of hydrophobic compounds by inorganic nano-ions: an unconventional mechanism. Journal of Colloid and Interface Science (2023), 638, 561–568. DOI: 10.1016/j.jcis.2023.01.115
[4] M. Hohenschutz, P. Bauduin, C. G. Lopez, B. Förster and W. Richtering. Superchaotropic Nano-ion Binding as a Gelation Motif in Cellulose Ether Solutions. Angewandte Chemie International Edition (2022), 16, e202210208. DOI: 10.1002/anie.202210208