
Publication in npj Materials Degradation
Team L2MEFor the first time, L2ME researchers have demonstrated how the nature of actinide colloids influences the colloid-sensitizer interactions and how these interactions condition the mesostructure of the final actinide oxide nanoporous material. The strong reactivity of tetravalent actinide ions such as thorium in aqueous media leads to uncontrolled and irreversible condensation and thus to the formation of colloids with varying sizes. Here, by the addition of a complexing agent such as formic acid, we were able to prepare sols containing colloids of controlled size and uniform degree of condensation exhibiting strong interactions with a carboxylic-functional surfactant. This led to the formation and preservation of a sufficiently rigid mesophase after thermal treatment, which allowed us to obtain micrometric nanofibers of ThO2 with accessible nanoporosity. These first results are of interest for the synthesis of nanoporous materials from transition metals, lanthanides, and actinides by colloidal "soft-template" route and open new perspectives for the fabrication of actinide oxides impregnable by minor actinide solutions for the preparation of homogeneous MOX nuclear fuel and the transmutation of minor actinides.
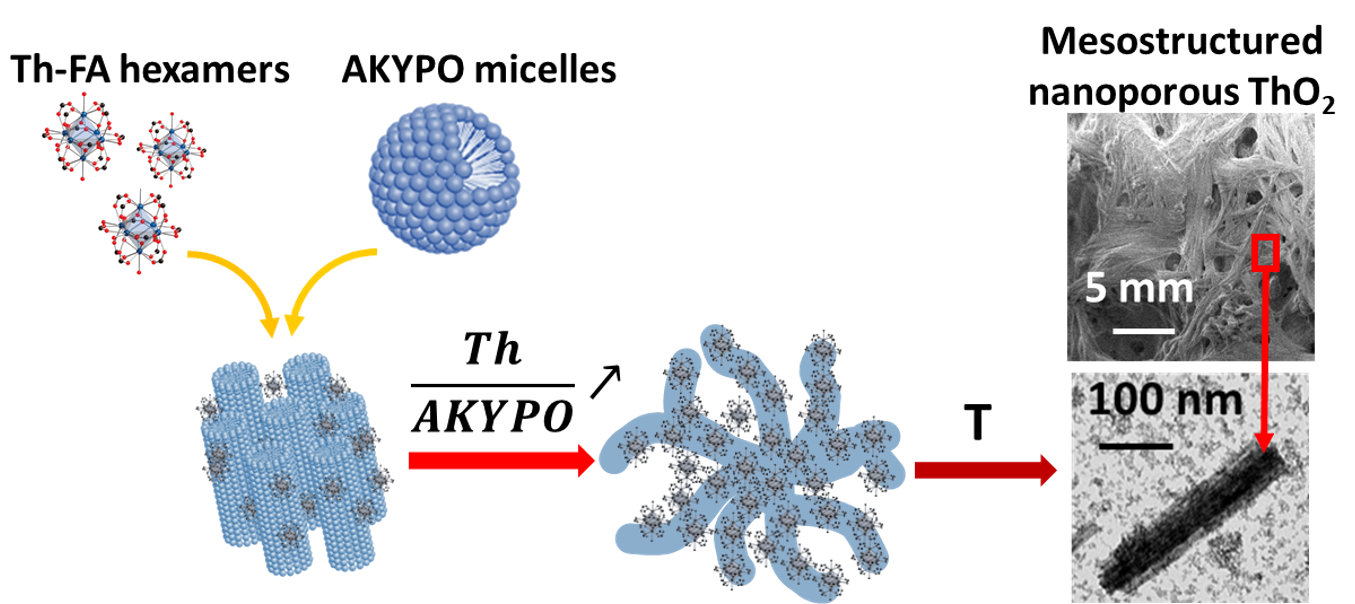
Credit: D. Rébiscoul / ICSM
For more information, read the article by Zijie Lu, Thomas Zemb, Xavier Le Goff, Joseph Lautru, Hassan Khoder, and Diane Rébiscoul in Journal of Colloid and Interface Science 637, 207 (2023). DOI: 10.1016/j.jcis.2023.01.087