
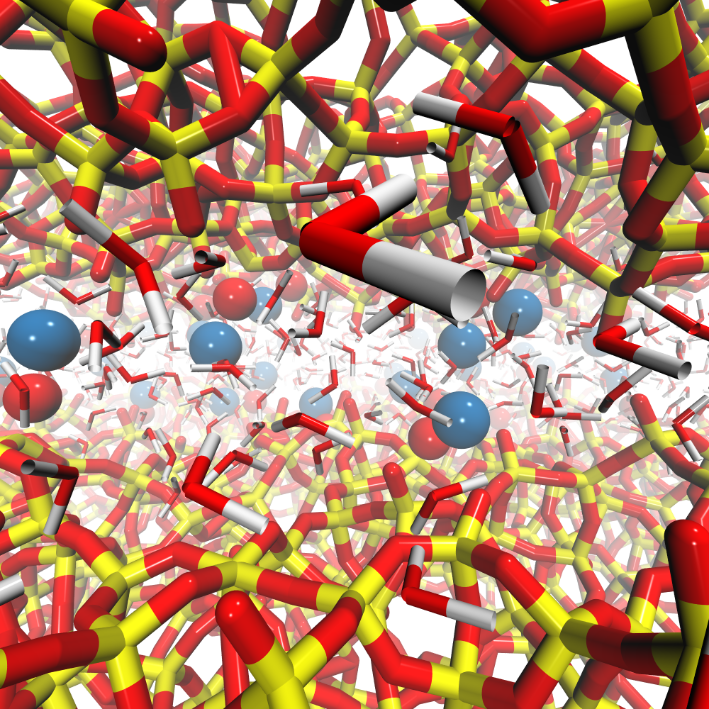
Confinement induces special properties on liquids: diffusion coefficients, viscosity, freezing points, and all collective properties which rest on the hypothesis of a minimal number of molecules in identical conditions, are affected by the vicinity of surfaces. Modelling offers a unique possibility to tackle these original properties. It allows for analyzing the origin of these new properties.
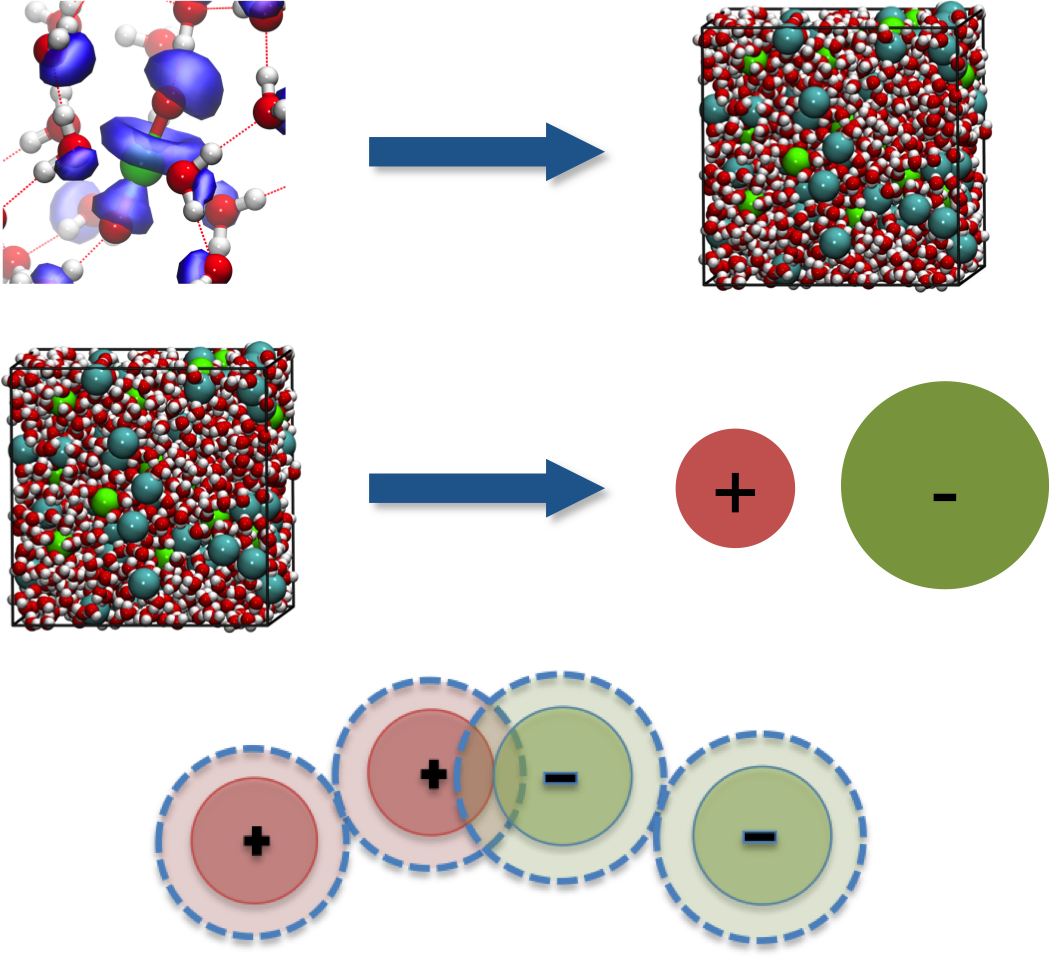
Ions in solution play a fundamental role in many physical, chemical, and biological processes. A multi-scale coarse graining procedure has been developped to derive such models from atomistic descriptions.
Effective (McMillan-Mayer) ion-ion potentials are derived from molecular dynamics simulations, allowing for defining an implicit solvent model of electrolytes. Then, perturbation calculations are performed to define the best possible representation for these systems, in terms of charged hard-sphere models.
Our final model is analytical and contains no free “fitting” parameters. It shows good agreement with the exact results obtained from Monte-Carlo simulations for the thermodynamic and structural properties.
Development of a similar model for the electrolyte viscosity, from information derived from atomistic descriptions, is also introduced.
The mesoscopic modelling of the microemulsion (water / oil / surfactant) thermodynamics properties is crucial to understand the phenomena occuring during the liquid-liquid extraction process (mainly used for the ions separation).
The mains objectives of this study are :
Collaboration with L. Arleth (University of Copenhagen, Danemark), S. Marcelja (Australian National University, Canberra)
Part of the film (Youtube video) shown on the CEA Saclay's 3D video wall