

The experiments dedicated to the solid/solid interfaces are mainly devoted to preparation of inorganic powder samples with tailored properties (morphology, size, composition, …) and the study of their sintering.
In this aim, the first step of our studies generally dealt with the preparation of mixed oxides as models for nuclear fuels (Th1-xUxO2, Th1-xCexO2, U1-xCexO2-y, Ce1-xNdxO2-x/2, Th1-xNdxO2-x/2, …). Wet chemistry methods based on the initial precipitation of crystallized precursors were generally considered for the synthesis, in order to improve the reactivity and the sintering capability of the prepared powders, and to homogenize the cationic distribution. In parallel to the synthesis of actinides-bearing molecular complexes, a particular attention was paid to the direct precipitation of hydrated oxides with a controlled morphology, especially using hydrothermal conditions. These studies particularly led to the preparation of actinides oxides microspheres with a hierarchical architecture, and of various compositions of (AnIV,Ln)O2 nanocrystals (An = Th, U).
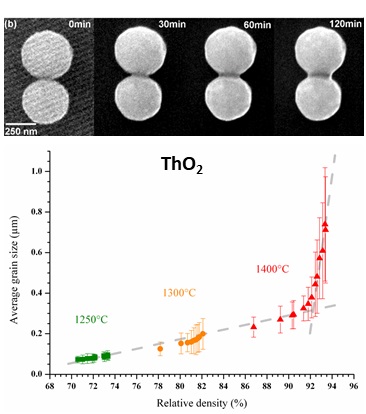
Using this large set of samples, the sintering of Th1-xUxO2, Th1-xCexO2, U1-xCexO2-y, Ce1?xNdxO2?x/2 and Th1?xYxO2?x/2 was studied in order to point out the powder-related parameters (specific surface area, homogeneity, amount of residual carbon, …) that can impact significantly the densification process. The thermal history of a solid, which includes the precursor chosen for the preparation, its conversion and the final microstructure of the sintered pellet, was then described. The establishment of sintering maps, which can be used to control in fine the microstructure of ceramics, was undertaken for several materials of interest. Also, in situ HT-ESEM observations led to the first experimental data concerning the kinetics and thermodynamics of the first step of UO2 sintering (elaboration of necks between the grains).
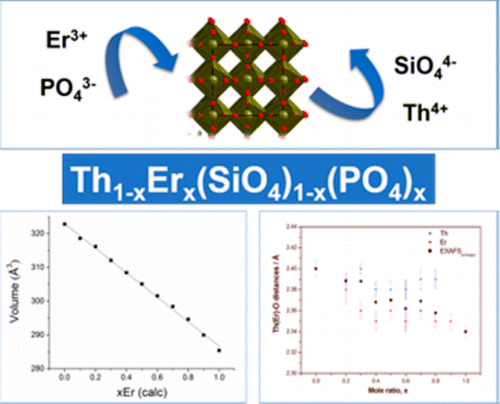
Several phosphate-based compounds were envisaged as host phases for actinides storage (including rhabdophane, monazite and xenotime). The preparation of this compounds consists in the incorporation of tetravalent actinides through different coupled substitution, i.e. 2 LnIII ⇔ AnIV + MII (monazite/cheralite solid solutions) or LnIII + PO4 ⇔ AnIV + SiO4 (monazite/huttonite solid solutions). Preparation of these samples was achieved for the first time with An = Th using a wet-chemistry route, while the synthesis of U-based samples is now under progress. This study has been developed on the xenotime/thorite system within the ANR X-MAS project (2018-2020).
The study of the solid/liquid interface mainly took place in the frame of spent fuel reprocessing, and of actinides wasteforms long-term behavior. More recently, this scope was also extended to the direct storage of spent fuel in underground repository.
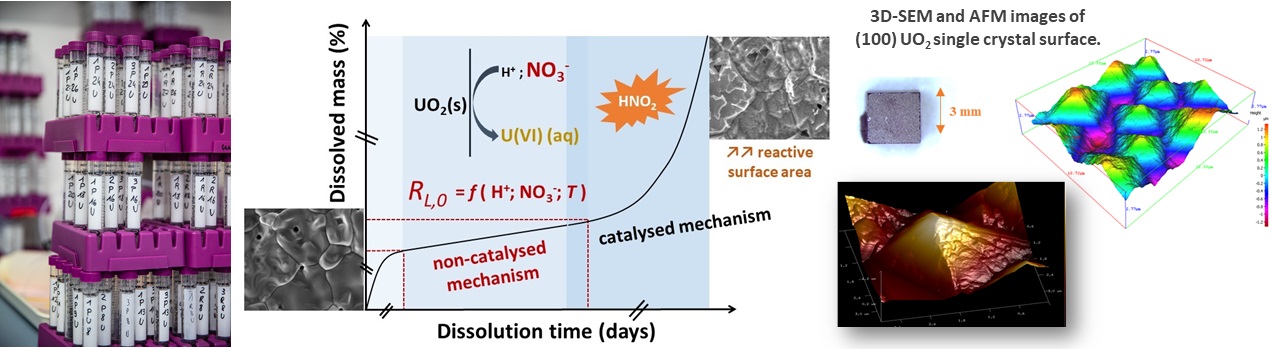
Studies regarding the reprocessing of spent fuel were mainly based on multi-parametric dissolution tests. For Th1-xUxO2 solid solutions, the modification of the driving dissolution mechanism (i.e. oxidation vs. surface reactions) as a function of the uranium content and the media acidity was evidenced both at the macroscopic and the microscopic scale, using operando ESEM observations. As such, links were established between the microstructure of the materials and their chemical durability. Moreover, the catalytic role of chemical species produced during the dissolution of these samples in nitric media was studied. In parallel, the transition from a catalyzed to non-catalyzed dissolution regime was observed in the case of UO2 single crystals, which allowed to avoid any bias coming from the microstructure. In a similar way, the presence of platinum group metals (PGM) elements within UO2 was investigated. Such experiments revealed the huge role of catalytic phenomena linked to the presence of PGM elements in the solid and in solution.
Each step of the nuclear fuel cycle involves chemical processes governed by thermodynamics. The “front end” of the nuclear fuel cycle includes mining of uranium or thorium ores, their processing and purification, and fuel fabrication. The “back end” corresponds to the direct disposal or reprocessing of the “spent fuel” and to the conditioning and disposal in an appropriate repository of the nuclear wastes.

Understanding the behavior of U or Th in these processes relies on thermodynamic calculations, which requires well constrained thermodynamic data. In order to determine consistent sets of thermodynamic data, the LIME group developed a methodology based on the synthesis of single-phased compounds associated to solubility measurements and oxide melt calorimetry (in collaboration with Univ. Davis, California and Washington State Univ.). This methodology was applied to coffinite and uranothorite solid-solutions, Th1-xUxSiO4, then to phosphates like rhabdophanes and monazites.