
New results (01/10/2025)
Team LTSMThe LTSM has identified and clarified the microscopic mechanisms leading to demixing of the organic phase in liquid–liquid extraction. In the case of uranium extraction by trioctylamine diluted in linear alkanes, the microstructures of the light phase and the third phase were characterized using a combined SAXS and SANS approach.
The third phase consists of water-in-oil aggregates exhibiting interdigitated hydrocarbon chains. The SANS experiments reveal the presence of residual molecules of deuterated diluent. The light phase mainly contains monomeric amines and amine aggregates associated with water. In the third phase, only a fraction of the aggregates incorporate uranyl(VI) cations. The uranium-loaded aggregates exhibit stronger attractive interactions than the uncharged ones. The demixing therefore results from their segregation into large domains, as evidenced by a Porod-type decay in SAXS at low q values.
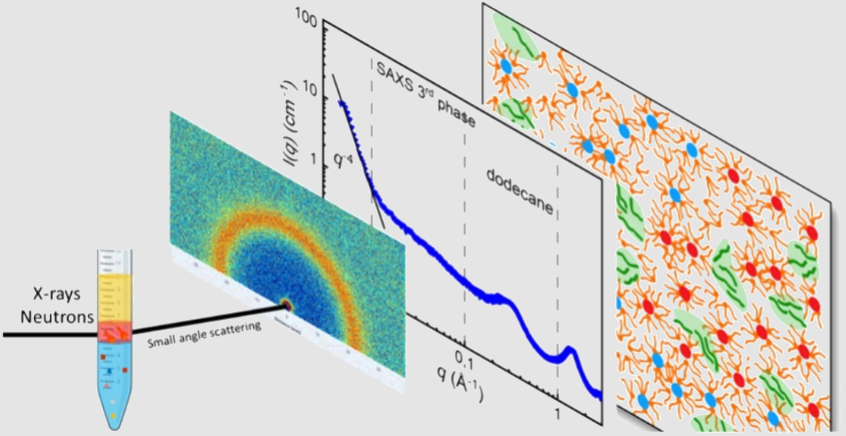
Credit: ICSM/LTSM
In conclusion, these results illustrate that liquid–liquid extraction is not limited to a simple ionic transfer between two phases, but relies on a complex supramolecular organization, in which the structuring of aggregates and their interactions govern phase stability and process performance. This understanding will make it possible to better grasp the mechanisms underlying instabilities in liquid–liquid extraction systems, to anticipate the occurrence of third phases, and to optimize solvent formulations in order to enhance the robustness and efficiency of separation processes.