
New results (03/09/2024)
Team L2IAProteins play an essential role in living organisms, most often within protein self-assemblies. Compared to free proteins, these are more stable. The ability to control these assemblies is therefore crucial for biotechnologies.
With this in mind, researchers at the Institut de Chimie Séparative de Marcoule (ICSM) and CEA-Joliot (Li2D) have studied the adsorption properties of nanoscale, low charge density inorganic ionic species (nano-ions) on different proteins.
More specifically, they observed the role of anionic boron clusters – COSAN- or halogenated (X = Br, I) dodecaborates (B12X122-) – on three proteins (myoglobin, carbonic anhydrase and trypsin inhibitor). They show that, remarkably, nano-ions lead to the reversible formation of 2D protein assemblies, without affecting protein structure. What's more, the size of the assemblies can be adjusted simply by varying the nano-ion concentration. This appears to be a general phenomenon, as it exists for the three proteins studied, all of which have very different structures. The myoglobin assemblies obtained are resistant to heat denaturation up to 70°C.
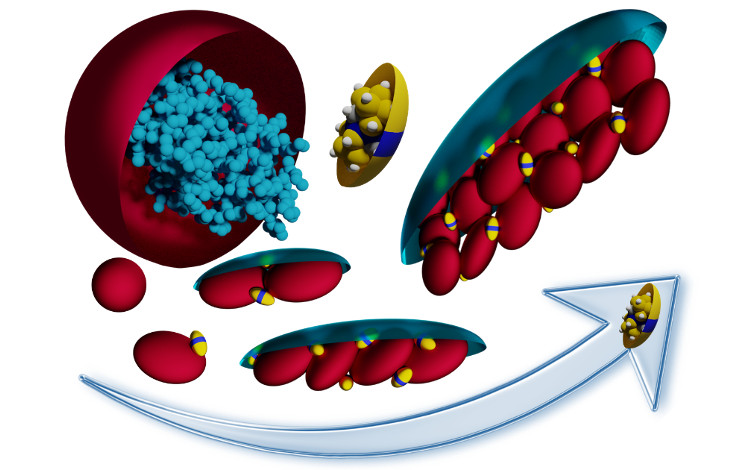
Credit: ICSM/L2IA
These results open up new applications for boron-based nano-ions in biotechnology and medicine for the construction and stabilization of protein assemblies. In particular, they shed new light on a Spanish study which demonstrated a cytostatic effect of COSAN (reversible blocking of cell division) and led to a patent for the preservation of cells and organs intended for transplantation. It therefore seems that the ability of nano-ions to form protein assemblies and prevent protein denaturation can be exploited to maintain protein functions under extreme conditions.
This work is part of the ProMeNIX (ANR-22-CE06-0026) and ChaoPOM (ANR-22-CE92-0043) projects, initiated by the Biocard2 projects (DRF Impulsion 2018) and the CEA Carbo-trans project (Bottom-Up 2019 exploratory program).
More information:
Controlling Protein Assembly with Superchaotropic Nano-Ions.
I. Chazapi, T. Merhi, C. Pasquier, O. Diat, Ch. Almunia, P. Bauduin. Angewandte Chemie International Edition (2024), In Press. DOI: 10.1002/anie.202412588