from the LSFC team and on the following topic:
"Synthesis, (sono)chemical reactivity, and structural characterization of actinide peroxides (U, Pu)"
Defense scheduled for Wednesday, December 11, 2024 at 1:30 PM (ICSM Auditorium).
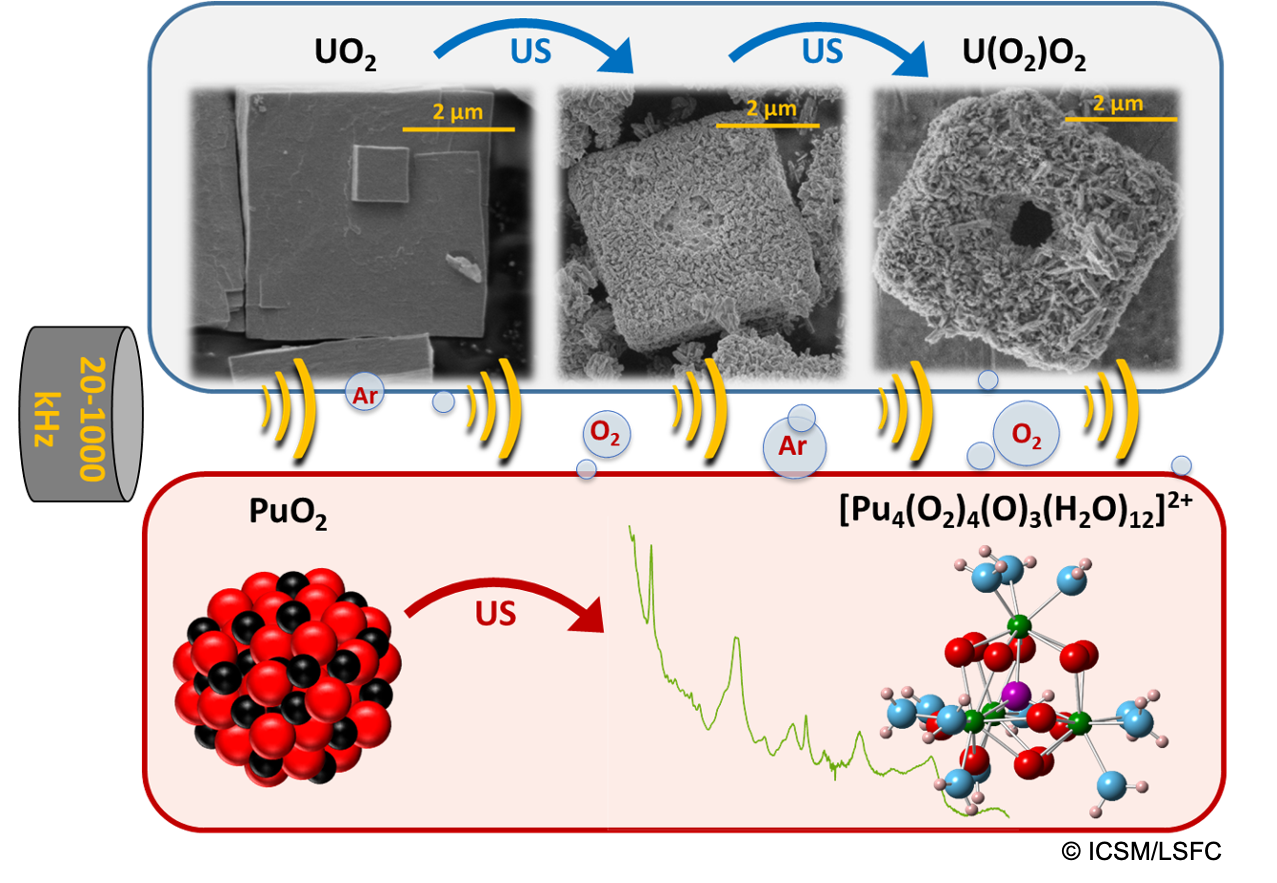
Although hydrogen peroxide (H2O2) has been widely used in nuclear chemistry for over 75 years, the preparation and literature description of actinide peroxides, particularly for uranium and plutonium, remain surprisingly rare. These two elements play a crucial role in the field of nuclear energy, and a thorough understanding of their properties when in contact with H2O2 is of fundamental, environmental, and industrial interest. In this context, the behavior of uranium and plutonium in solution and in oxide form has been studied in the presence of H2O2 in diluted aqueous solution. Sonochemistry, which investigates the effect of ultrasound waves on chemical reactions, has been used to generate H2O2 in situ and in a controlled manner. Under an Ar/O2 atmosphere and at high ultrasonic frequency, it has been shown that uranyl peroxide (studtite or metastudtite) can be formed from uranyl solutions or uranium oxide (UO2). In particular, under certain experimental conditions, characterizations revealed an original phenomenon of pseudomorphicconversion, with the possible emergence of a central hole in the structure. The study of the involved mechanism suggested complex sonochemical processes that complement the existing literature on the behavior of materials under sonochemical stress. Meanwhile, the sonochemical behavior of nanometric plutonium dioxide (PuO2), in powdered or colloidal form, highlighted significant differences in reactivity depending on their surface state. Notably, the interaction of colloidal PuO2 nanoparticles with H2O2 was particularly interesting, leading to the formation of a new plutonium peroxide. This compound was studied and characterized in detail using laboratory analytical techniques and synchrotron radiation, revealing an original polynuclear structure of Pu(IV) that enriches the limited knowledge of tetravalent actinide peroxides.
Keywords: Actinide peroxide; Sonochemistry; Uranium and Plutonium; Chemical reactivity; Polynuclear structure