
article in Angewandte Chemie, International Edition
Equipe L2IA
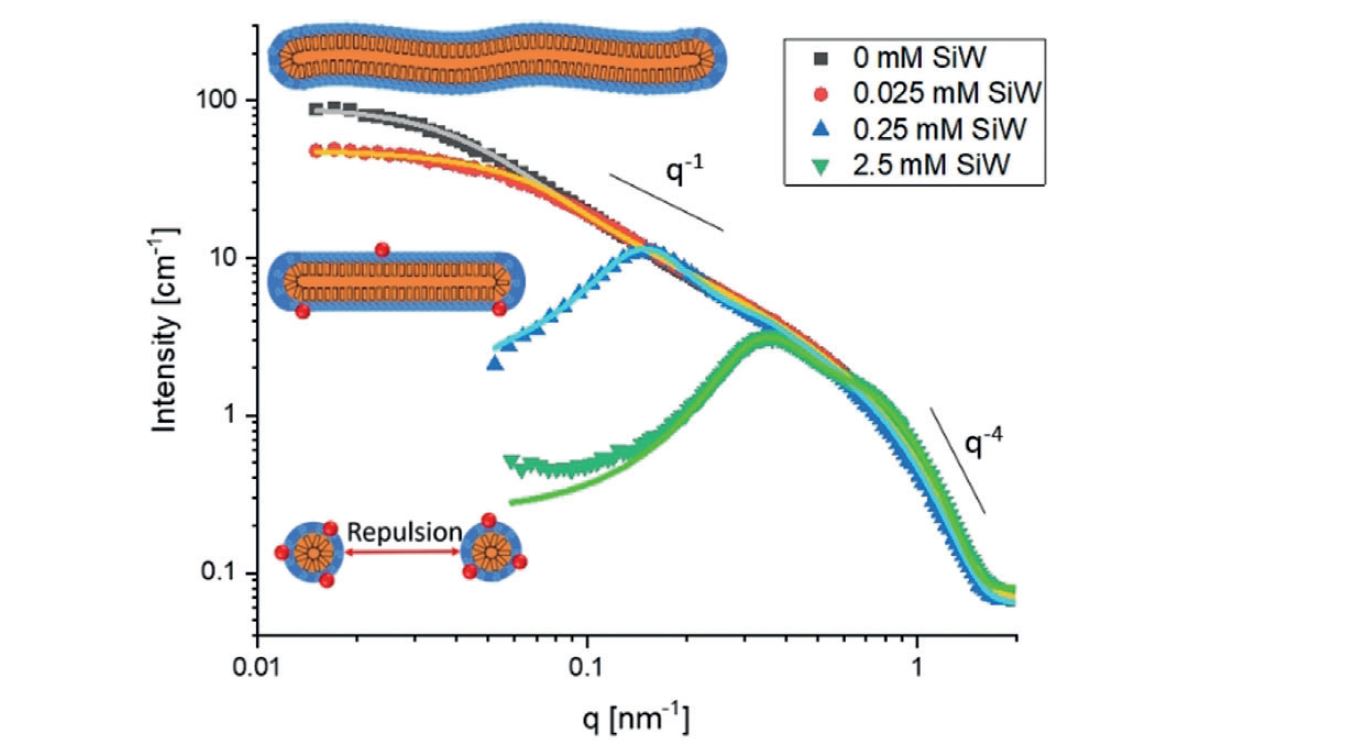
Some recent studies have highlighted that nanometer-sized ions characterized by a low charge density have a strong propensity to adsorb to hydrated neutral surfaces or to bind to macrocyclic molecules cavities. This ion specific effect and named “superchaotropicity” in reference to the more classical chaotropic behavior arises from a solvent mediated effect. Investigating phase diagrams and analyzing small angle neutron scattering curves, we have shown that adding various salts of nano-ions to different mesophases of a non-ionic surfactant induces i) the disappearance of the Liquid/liquid micellar diphasic regime versus T, ii) the spontaneous transformation of the cylindrical micelles in spherical ones and in strong repulsive interaction, iii) the spontaneous transformation of a swollen lyotropic lamellar phase into a vesicle phase. This effect already observable by adding ionic surfactants are also measurable at very low concentration in the range of few micromolar. However, contrarily to ionic surfactants, nano-ions dehydrate strongly the neutral surfactant assemblies and thus charges screening action via adding more classical salts will generate new condensed structures.
Lire l'article Hohenschutz Max, Grillo Isabelle, Diat Olivier and Bauduin Bauduin,, Angewandte Chemie, International Edition 59 (2020) accepted DOI: 10.1002/anie.201916193 and 10.1002/ange.201916193